Where is APC going?
(JCB 154:1105-1109, 2001)
Yuko Mimori-Kiyosue1,2 and Shoichiro Tsukita2,3
Abstract: APC (adenomatous polyposis coli) protein has been
thought to be involved in tumor suppression through the Wnt/b-catenin
signaling pathway. However, its connections to the cytoskeleton,
especially microtubules, are becoming apparent, and the new functions
of APC are attracting increasing interest in understanding the
role of APC not only in tumorigenesis, but also in normal physiology.
Defects in the APC (adenomatous polyposis coli)
tumor suppressor gene is linked to the development of colorectal
cancer (reviewed in Nakamura, 1993; Kinzler and Vogelstein, 1996).
Somatic mutations in the APC gene occur in the majority
of sporadic colorectal tumors, and germline mutations in APC
are responsible for familial adenomatous polyposis, an autosomal
dominant inherited disease. The APC protein (APC) is a large multi-domain
protein with a molecular mass of 300 kD (Fig.1). 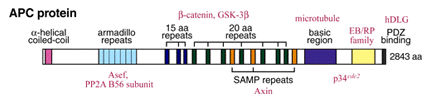 APC’s modular architecture led to attempts to identify
binding partners for each domain, and the discovery that APC binds
to b-catenin, a protein that functions in cell adhesion as well
as in Wnt-based signal transduction, provided the first significant
insight into the biological function of APC in molecular terms
(Rubinfeld et al., 1993; Su et al., 1993).
APC’s modular architecture led to attempts to identify
binding partners for each domain, and the discovery that APC binds
to b-catenin, a protein that functions in cell adhesion as well
as in Wnt-based signal transduction, provided the first significant
insight into the biological function of APC in molecular terms
(Rubinfeld et al., 1993; Su et al., 1993).
Since then, there have been numerous exciting findings regarding
the functional relationship between APC and Wnt signaling (reviewed
in Cadigan and Nusse, 1997; Peifer and Polakis, 2000). On the
other hand, little attention was paid to the subcellular localization
of APC. However, another important functional aspect of APC has
been abruptly highlighted; its role in association with cytoskeletons.
In these studies, of course, the localization of APC has been
examined in detail. In this mini-review, we will overview this
newly emerging field in the study of APC, paying special attention
to its subcellular distribution.
APC at cell-cell adhesion sites
In various polarized epithelial cells, APC has been found at the
sites of cell-cell adhesion. In normal mouse intestinal epithelial
cells, immunoelectron microscopy showed APC to be localized in
the cytoplasm with a significant concentration along the lateral
plasma membrane (Miyashiro et al., 1995). The observation that
the COOH terminus of APC directly binds to the PDZ domain of hDLG,
a human homologue of the Drosophila discs large (DLG)
tumor suppressor protein, also favored the notion that APC is
associated with cell-cell adhesion sites and/or lateral membranes
(Matsumine et al., 1996), since hDLG is distributed along lateral
membranes. Also in several cultured cell lines, APC showed some
concentration at cell-cell contact sites (Nathke et al., 1996;
Fig.2B). 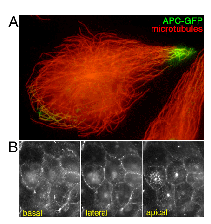
The occurrence of multiple APC proteins in a single species has
been reported recently (reviewed in Dikovskaya et al., 2001);
APC and APC2/APCL with a smaller molecular mass in human and mouse,
and dAPC and dAPC2/E-APC in Drosophila. Their central portion
containing armadillo repeats and the b-catenin binding region
is fairly conserved, but their COOH-terminal region diversifies
significantly. Consequently, in Drosophila, both dAPC and
dAPC2/E-APC lack the PDZ-binding motif at their COOH termini,
suggesting that they do not interact with DLG. However, recent
studies suggested a functional relationship between Drosophila
APC and cell-cell adhesion. In Drosophila, dAPC2/E-APC,
which is ubiquitously expressed and abundant in epithelial cells,
was found to be concentrated at the apicolateral adhesive zones
of epithelial cells (McCartney et al., 1999). This junction-specific
concentration required an intact actin cytoskeleton, and depletion
or mutation of dAPC2/E-APC in embryos resulted in partial defects
in the recruitment of Armadillo, a Drosophila homologue
of b-catenin, to the junctions (Yu et al., 1999; Townsley and
Bienz, 2000). These observations suggest a role for Drosophila
APC in the genesis and maintenance of the integrity of cell-cell
junctions.
APC on microtubules
In addition to immunohistochemical analyses, overexpression
experiments was also conducted to determine the intracellular
localization of APC in several cell lines (Munemitsu et al., 1994;
Smith et al., 1994). In these studies, unexpectedly, the exogenously
expressed wild-type APC appeared to be distributed along microtubules.
In agreement with this, APC was shown to directly bind to microtubules
at its COOH-terminal basic region and to stabilize microtubules
in vitro (Munemitsu et al., 1994), as well as
in vivo (Zumbrunn et al., 2001), similarly to conventional
MAPs (microtubule-associated proteins). Subsequently, a high-resolution
immunofluorescence study of endogenous APC in cultured MDCK cells
was performed (Nathke et al., 1996). In these cells, endogenous
APC was localized in clusters of puncta near the ends of microtubules
at peripheral membrane sites of migrating edges, and this localization
appeared to require intact microtubules. However, as a great deal
of attention has been focused on the function of APC that pertains
to its ability to regulate b-catenin activity, despite Nathke's
beautiful images, the relationship of APC to microtubules has
been mostly neglected. However, movies were more influential than
still images. Analysis of GFP (green fluorescent protein)-tagged
APC (APC-GFP) in living Xenopus A6 epithelial cells uncovered
a peculiar dynamic behavior of APC within cells (Mimori-Kiyosue
et al., 2000a): APC-GFP moved continuously along a subset of microtubules
toward their distal ends in an energy-dependent manner and accumulated
as granular aggregates at the ends (Fig.2A). These movies, together
with the results of other concurrent genetic and immunofluorescence
studies on APC, prompted many APC researchers to turn back to
microtubules (reviewed in McCartney and Peifer, 2000).
Microtubule search-and-capture mechanism
Microtubules are structurally very dynamic, and their distal (plus)
ends are the primary sites of growth and shortening, exhibiting
dynamic instability (reviewed in Desai and Mitchison, 1997). Microtubule
organization is highly polar, and microtubule dynamics vary considerably
between different regions of the cell and stages of the cell cycle,
suggesting that they are spatially and temporally controlled.
Through intensive observations of such dynamic behavior of microtubules,
Kirschner and Mitchison proposed a ‘search-and-capture’ mechanism:
during interconversion between growth and shortening of their
plus ends, microtubules search for the sites (e.g. plasma membranes,
chromosomes, organelles etc.) with which they interact to capture,
followed by stabilization and reorientation of the microtubule-based
cytoskeleton (1986) (Fig.3A). However, at that time, the molecular
components involved in this search-and-capture mechanism remained
undefined. 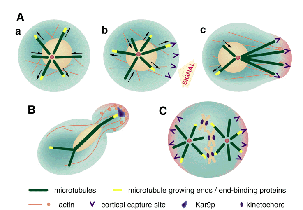
In recent years, mainly using GFP technology, several proteins,
that are specifically concentrated in transient segments at the
growing plus ends of microtubules, have been identified (Table
1). These proteins are thought to be copolymerized into plus ends
of microtubules, where they remain for a while, and then dissociate
from microtubules, allowing the existence of specialized transient
segments at the plus ends of microtubules only in the growth phase
(reviewed in Sawin, 2000; Schroer, 2001; Schuyler and Pellman,
2001). These findings naturally led to the hypothesis that these
transient segments (and also proteins specifically associated
with these segments) play a crucial role in the search-and-capture
mechanism of microtubules. Interestingly, from the viewpoint of
the APC study, EB1, which was identified as an APC binding protein
by yeast two-hybrid screening, was also included in this category
of proteins (Su et al., 1995; Mimori-Kiyosue et al., 2000b, reviewed
in Tirnauer and Bierer, 2000).
Recent studies in yeast showed that EB1 acts as a crosslinker
between microtubule ends and the cell cortex. Bim1p, a budding
yeast homologue of EB1, was identified as a tubulin-binding protein
whose deletion causes defects in orienting spindles (Schwartz
et al., 1997). In the budding yeast, the spindle microtubules
search for and capture the tip of daughter buds to align spindles
and thereby segregate the nucleus correctly (Fig.3 B). Genetic
analyses have revealed that spindle orientation requires several
polarity proteins that localize to the tip of buds, including
Kar9p which was originally identified in a screen for karyogamy
mutants as a protein involved in nuclear migration (Miller and
Rose, 1998). Interestingly, Bim1p was found to directly interact
with Kar9p and recruit it to microtubules. (Lee et al., 2000;
Korinek et al., 2000). Therefore, it is now believed that Bim1p
on the plus ends of microtubules captures Kar9p at the tips of
buds to assist spindle orientation and faithful cell division.
Intriguingly, EB1 is conserved in a wide range of organisms from
yeast to human, but no Kar9p homologues have yet been found in
other species. This raises the question of the identity of the
counterpart of Kar9p in vertebrates. Taking the affinity between
EB1 and APC as well as the subcellular localization of APC, it
is tempting to speculate that APC is one of the functional counterparts
of Kar9p in multicellular organisms. Of course, this speculation
should be evaluated experimentally in more detail, since EB1 and
APC show no structural similarity.
APC during mitosis
If APC is one of counterparts of Kar9p, it is likely that,
also in higher organisms, the APC-EB1-based microtubule-capturing
process occurs during cell division (Fig.3C). This led Jan and
colleagues (Lu et al., 2001) to test the function of dAPC2/E-APC
in epithelial cell division in Drosophila embryos. Drosophila
neuroepithelial cells divided in a symmetric manner, but when
adherens junctions were destroyed, they changed to divide in an
asymmetric manner. Similarly, depletion of dAPC2/E-APC or Drosophila
homologue of EB1 (dEB1) by the RNA interference (RNAi) method
induced asymmetric cell division. Although the direct binding
between dAPC2/E-APC and dEB1 was not detected in vitro,
these findings suggested the involvement of dAPC2/E-APC and dEB1
in determination of spindle orientation. Moreover, in dividing
neuroblasts, dAPC2/E-APC was shown to be asymmetrically localized
at the cortex in a crescent adjacent to one spindle pole (McCartney
et al., 1999), suggesting the involvement of dAPC2/E-APC also
in asymmetric cell division.
Recently, two independent groups reported that in mitotic cells
APC is localized at kinetochores, the microtubule attachment sites
of chromosomes, and that APC mutant cells are defective in spindle
formation and chromosome segregation (Kaplan et al., 2001; Fodde
et al., 2001). Furthermore, Kaplan et al. showed that APC forms
a complex with cell-cycle checkpoint proteins, Bub1 and Bub3,
at kinetochores, and proposed a model in which APC monitors the
accurate attachment of microtubule ends to kinetochores. These
findings led to the intriguing hypothesis that APC (and probably
EB1) is essential for microtubules to search for and capture the
kinetochores during cell division. In the contrast, it was reported
that the APC-EB1 interaction is down-regulated in mitotic cells
(Askham et al., 2000). Therefore, the above hypothesis should
be evaluated experimentally in future studies.
APC during cell migration
The search-and-capture mechanism of microtubules could also
work in migrating cells. During migration, microtubules are asymmetrically
organized facing their plus ends toward the leading edge of the
cell. When the wound healing process was observed using A6 cells
expressing GFP-APC, at the front row of the wound GFP-APC began
to gradually concentrate at the distal ends of a subset of microtubules,
which appeared to grow continuously toward the wounded region
(Mimori-Kiyosue et al., 2000a). EB1 appeared to be colocalized
with APC only at these ends of microtubules (Mimori-Kiyosue et
al., 2000b). Furthermore, APC appeared to associate with microtubules
preferentially in migrating epithelial cells, but not in highly
polarized cells (Nathke et al., 1996) (Fig.2B). These findings
suggested that in migrating cells the APC-EB1 interaction plays
some important role in guiding microtubule plus ends to specific
cortical sites, as observed in the tips of daughter buds of yeasts.
Recently, the intimate relationship between APC and the actin-based
cytoskeleton was also discussed. Asef, APC-stimulated guanine
nucleotide exchange factor (GEF), was identified as one of the
binding partners for the armadillo repeat region of APC (Kawasaki
et al., 2000). APC was shown to enhance the GEF activity of Asef,
resulting in the activation of Rac, a small G protein, followed
by actin polymerization at the cell periphery, i.e. membrane ruffling,
and lamellipodia formation, in MDCK cells. This finding may provide
an important clue to understand how APC is involved in the regulation
of not only microtubule-based but also actin-based cytoskeletons.
APC in nucleus, apical membranes, ------
We should point out that there has been some controversial
reports on the subcellular localization of APC. For example, APC
was reported to be localized in the nucleus in several cellular
systems (e.g. Neufeld and White, 1997), but this nuclear localization
still remains somewhat controversial (Nathke et al., 1996). Furthermore,
APC was recently reported to be concentrated at apical plasma
membranes in a variety of polarized epithelial cells (Reinacher-Schick
and Gumbiner, 2001), but this appears inconsistent with most of
the previous observations. Indeed, in highly polarized A6 cells
expressing APC-GFP no intense signal was detected from apical
membranes (Fig.2B), although exogenously expressed APC-GFP in
culture cells does not always mirror the behavior of endogenous
protein. In general, we have often encountered difficulty in detection
of endogenous APC molecules by immunofluorescence microscopy,
partly due to the low expression level of endogenous APC, and
partly due to problems in the specificity of commercially available
antibodies. After careful consideration, we should discuss where
APC will go in the future study.
Where will APC go?
In recent years, based on the subcellular localization and
dynamic behavior of APC, several new aspects of APC have emerged.
Interestingly, most of these new aspects appear to be related
to the cytoskeleton, in particular, the organization of microtubule
networks. In this mini-review, we presented an overview of these
new findings along with the search-and-capture mechanism of microtubules.
This scenario, especially the relationship between APC and Kar9p,
should be evaluated experimentally in more detail, but the scenario
discussed here may be helpful for researchers to appreciate that
the challenge of answering many questions lies ahead before understanding
the whole picture of APC functions.
Patients suffered from familial adenomatous polyposis develop
hundreds to thousands of polyps in the colon and rectum at an
early age, a subset of which invariably progress to malignant
cancers if not surgically removed. Polyp formation is initiated
by abnormal accumulation of the intestinal epithelium at the crypt-villus
boundary, where, in the normal intestine, enterocytes migrate
up toward the tips of villi maintaining the integrity of a tight
layer of cells with concomitant differentiation. These findings
suggested the possible involvement of APC not only in the proliferation
and differentiation but also in migration and adhesion of epithelial
cells (reviewed in Hansken et al., 1994; Polakis, 1997; Bienz
and Clevers, 2000). Ā@Indeed, as discussed above, evidence is now
accumulating that APC plays a crucial role in cellular migration
and adhesion through its associations with the cytoskeleton. Therefore,
further analyses of these newly-identified functions of APC will
lead to a better understanding of the molecular mechanism of the
APC-based tumorigenesis.
From the viewpoint of cancer research, the idea is also intriguing
that APC is directly involved in chromosome segregation as well
as in microtubule orientation during mitosis. This is particularly
attractive when considering the genetic instability and the loss
of epithelial polarity during tumorigenesis. In APC mutant
mice, intestinal adenomas are polyclonal during the early stage
of polyp formation, and this polyclonality appears to be responsible
for tumor progression (Merritt et al., 1997). Actually, human
colorectal cancer cells were shown to exhibit a marked defect
in chromosome segregation (Lengauer et al., 1997). These observations
could be explained by the chromosomal instability induced by miss-searching
and/or miss-capturing of kinetochores in dividing cells due to
APC mutations. On the other hand, the aberrant orientation of
the cell-division plane, which also could be induced by APC mutations,
may affect the distribution of cells in the intestine, resulting
in the loss of normal monolayer organization. Nevertheless, these
ideas are still largely speculative, and thus further experimental
support is needed to consolidate this presumptive role of APC
in cell division as well as tumorigenesis.
Finally, we should discuss the relationship of the new functions
of APC summarized in this review to its ‘classic’ function, i.e.
the destruction of b-catenin. Several lines of evidence suggest
that the function of APC at the ends of microtubules is regulated
by Wnt/b-catenin signaling. Without Wnt signaling, wild-type b-catenin
is rapidly degraded and undetectable in the APC clusters at microtubule
ends. However, an exogenously expressed stable b-catenin mutant,
N-terminally truncated b-catenin (DN-b-catenin), accumulated in
the APC clusters. Moreover, expression of DN-b-catenin impaired
the migratory properties and formation of cellular extensions
of MDCK cells (Barth et al., 1997; Pollack et al., 1997). These
findings led to the speculation that increased stability of b-catenin
(and resultant accumulation of b-catenin in the APC clusters)
suppresses the APC function at microtubule ends, i.e. the extension
of cellular processes through stabilization of microtubules. It
has been widely accepted that APC regulates the function of b-catenin,
but this speculation implies the reverse, i.e. b-catenin regulates
the function of APC.
We now know much about the relationship between APC and the Wnt/b-catenin
pathway, but the interactions between APC and the cytoskeleton
are becoming apparent. The APC-based connections between the Wnt/b-catenin
pathway and cytoskeleton are not yet apparent, but are likely
to emerge in the coming years. We are only just beginning to seek
the missing pieces linking signal transduction to structure and/or
vice versa.
----------------------------------------------
We are grateful to Drs.H.Yamauchi and T.Nagasu (KAN Research Institute)
for continuous encouragement. Our thanks are also due to Drs.A.Nagafuchi
(Kumamoto Univ.), A.Kikuchi (Hiroshima Univ.) and H.Oda (ERATO)
for their critical reading of this manuscript.
Movies showing the dynamic behaviors of APC and EB1 are supplemented
in Mimori-Kiyosue et al. (2000a), Mimori-Kiyosue et al. (2000b),
and Eccleston (2001).
References
Askham,J.M., P.Moncur, A.F.Markham, and E.E.Morrison. 2000.
Regulation and function of the interaction between the APC tumor
suppressor protein and EB1. Oncogene 19:1950-1958.
Barth,A.I., A.L.Pollack, Y.Altschuler, K.E.Mostov, and W.J.Nelson.
1997. NH2-terminal deletion of b-catenin results in stable colocalization
of mutant b-catenin with adenomatous polyposis coli protein and
altered MDCK cell adhesion. J.Cell Biol. 136:693-706.
Bienz,M., and H.Clevers. 2000. Linking colorectal cancer to Wnt
signaling. Cell 103:311-320.
Cadigan,K.M., and R.Nusse. 1997. Wnt signaling: a common theme
in animal development. Genes Dev. 11:3286-3305.
Desai,A., and T.J.Mitchison. 1997. Microtubule polymerization
dynamics. Annu.Rev.Cell Dev.Biol. 13:83-117.
Dikovskaya,D., J.Zumbrunn, G.A.Penman, and I.S.Nathke. 2001. The
adenomatous polyposis coli protein:in the limelight out at the
edge. Trends in Cell Biology in press.
Eccleston,A. 2001. GFP in Motion 2. Trends in Cell Biology
11:311.
Fodde,R., J.Kuipers, C.Rosenberg, R.Smits, M.Kielman, C.Gaspar,
J.H.van Es, C.Breukel, J.Wiegant, R.H.Giles, and H.Clevers. 2001.
Mutations in the APC tumour suppressor gene cause chromosomal
instability. Nat.Cell Biol. 3:433-438.
Hansken,J., J.Behrens, and W.Birchmeier. 1994. Tumor-suppressor
gene products in cell contacts: the cadherin-APC-armadillo connection.
Curr.Opin.Cell Biol. 6:711-716.
Kaplan,K.B., A.A.Burds, J.R.Swedlow, S.S.Bekir, P.K.Sorger, and
I.S.Nathke. 2001. A role for the Adenomatous Polyposis Coli protein
in chromosome segregation. Nat.Cell Biol. 3:429-432.
Kawasaki,Y., T.Senda, T.Ishidate, R.Koyama, T.Morishita, Y.Iwayama,
O.Higuchi, and T.Akiyama. 2000. Asef, a link between the tumor
suppressor APC and G-protein signaling. Science 289:1194-1197.
Kinzler,K.W., and B.Vogelstein. 1996. Lessons from hereditary
colorectal cancer. Cell 87:159-170.
Kirschner,M., and T.Mitchison. 1986. Beyond self-assembly: from
microtubules to morphogenesis. Cell 45:329-342.
Korinek,W.S., M.J.Copeland, A.Chaudhuri, and J.Chant. 2000. Molecular
linkage underlying microtubule orientation toward cortical sites
in yeast. Science 287:2257-2259.
Lee,L., J.S.Tirnauer, J.Li, S.C.Schuyler, J.Y.Liu, and D.Pellman.
2000. Positioning of the mitotic spindle by a cortical-microtubule
capture mechanism. Science 287:2260-2262.
Lengauer,C., K.W.Kinzler, and B.Vogelstein. 1997. Genetic instability
in colorectal cancers. Nature 386:623-627.
Lu,B., F.Roegiers, L.Y.Jan, and Y.N.Jan. 2001. Adherens junctions
inhibit asymmetric division in the Drosophila epithelium.
Nature 409:522-525.
Matsumine,A., A.Ogai, T.Senda, N.Okumura, K.Satoh, G.H.Baeg, T.Kawahara,
S.Kobayashi, M.Okada, K.Toyoshima, and T.Akiyama. 1996. Binding
of APC to the human homolog of the Drosophila discs large
tumor suppressor protein. Science 272:1020-1023.
McCartney,B.M., H.A.Dierick, C.Kirkpatrick, M.M.Moline, A.Baas,
M.Peifer, and A.Bejsovec. 1999. Drosophila APC2 is a cytoskeletally-associated
protein that regulates wingless signaling in the embryonic epidermis.
J.Cell Biol. 146:1303-1318.
McCartney,B.M., and M.Peifer. 2000. Teaching tumour suppressors
new tricks. Nat.Cell Biol. 2:E58-60.
Merritt,A.J., K.A.Gould, and W.F.Dove. 1997. Polyclonal structure
of intestinal adenomas in ApcMin/+ mice with concomitant loss
of Apc+ from all tumor lineages. Proc Natl Acad Sci U S A.
94:13927-13931.
Miller,R.K., and M.D.Rose. 1998. Kar9p is a novel cortical protein
required for cytoplasmic microtubule orientation in yeast. J.Cell
Biol. 140:377-390.
Mimori-Kiyosue,Y., N.Shiina, and S.Tsukita. 2000a. Adenomatous
polyposis coli (APC) protein moves along microtubules and concentrates
at their growing ends in epithelial cells. J.Cell Biol.
148:505-518.
Mimori-Kiyosue,Y., N.Shiina, and S.Tsukita. 2000b. The dynamic
behavior of the APC-binding protein EB1 on the distal ends of
microtubules. Curr.Biol. 10:865-868.
Miyashiro,I., T.Senda, A.Matsumine, G.H.Baeg, T.Kuroda, T.Shimano,
S.Miura, T.Noda, S.Kobayashi, M.Monden et al. 1995. Subcellular
localization of the APC protein: immunoelectron microscopic study
of the association of the APC protein with catenin. Oncogene.
11:89-96.
Munemitsu,S., B.Souza, O.Muller, I.Albert, B.Rubinfeld, and P.Polakis.
1994. The APC gene product associates with microtubules in vivo
and promotes their assembly in vitro. Cancer Res. 54:3676-3681.
Nakamura,Y. 1993. The role of the adenomatous polyposis coli (APC)
gene in human cancers. Adv.Cancer Res. 62:65-87.
Nathke,I.S., C.L.Adams, P.Polakis, J.H.Sellin, and W.J.Nelson.
1996. The adenomatous polyposis coli tumor suppressor protein
localizes to plasma membrane sites involved in active cell migration.
J.Cell Biol. 134:165-179.
Neufeld,K.L., and R.L.White. 1997. Nuclear and cytoplasmic localizations
of the adenomatous polyposis coli protein. Proc.Natl.Acad.Sci.USA.
94:3034-3039.
Peifer,M., and P.Polakis. 2000. Wnt signaling in oncogenesis and
embryogenesis--a look outside the nucleus. Science 287:1606-1609.
Polakis,P. 1997. The adenomatous polyposis coli (APC) tumor suppressor.
Biochim.Biophys.Acta. 1332:F127-147.
Pollack,A.L., A.I.M.Barth, Y.Altschuler, W.J.Nelson, and K.E.Mostov.
1997. Dynamics of b-catenin interactions with APC protein regulate
epithelial tubulogenesis. J.Cell Biol. 137:1651-1662.
Reinacher-Schick,A, B.M.Gumbiner. 2001. Apical membrane localization
of the adenomatous polyposis coli tumor suppressor protein and
subcellular distribution of the beta-catenin destruction complex
in polarized epithelial cells. J.Cell Biol. 152:491-502.
Rubinfeld,B., B.Souza, I.Albert, O.Muller, S.H.Chamberlain, F.R.Masiarz,
S.Munemitsu, and P.Polakis. 1993. Association of the APC gene
product with b-catenin. Science 262:1731-1734.
Sawin,K.E. 2000. Microtubule dynamics: the view from the tip.
Curr.Biol. 10:R860-862.
Schroer,T.A. 2001. Microtubules don and doff their caps: dynamic
attachments at plus and minus ends. Curr.Opin.Cell Biol.
13:92-96.
Schuyler,S.C., and D.Pellman. 2001. Microtubule “plus-end-tracking
proteins”: The end is just the beginning. Cell 105:421-424.
Schwartz,K., K.Richards, and D.Botstein. 1997. BIM1 encodes a
microtubule-binding protein in yeast. Mol.Biol.Cell 8:2677-2691.
Smith,K.J., D.B.Levy, P.Maupin, T.D.Pollard, B.Vogelstein, and
K.W.Kinzler. 1994. Wild-type but not mutant APC associates with
the microtubule cytoskeleton. Cancer Res. 54:3672-3675.
Su,L.K., M.Burrell, D.E.Hill, J.Gyuris, R.Brent, R.Wiltshire,
J.Trent, B.Vogelstein, and K.W.Kinzler. 1995. APC binds to the
novel protein EB1. Cancer Res. 55:2972-2977.
Su,L.K., B.Vogelstein, and K.W.Kinzler. 1993. Association of the
APC tumor suppressor protein with catenins. Science 262:1734-1737.
Tirnauer,J.S., and B.E.Bierer. 2000. EB1 proteins regulate microtubule
dynamics, cell polarity, and chromosome stability. J.Cell Biol.
149:761-766.
Townsley,F.M., and M.Bienz. 2000. Actin-dependent membrane association
of a Drosophila epithelial APC protein and its effect on
junctional Armadillo. Curr.Biol. 10:1339-1348.
Yu,X., L.Waltzer, and M.Bienz. 1999. A new Drosophila APC
homologue associated with adhesive zones of epithelial cells.
Nat.Cell Biol. 1:144-151.
Zumbrunn,J., K.Kinoshita, A.A.Hyman, and I.S.Nathke. 2001. Binding
of the adenomatous polyposis coli protein to microtubules increases
microtubule stability and is regulated by GSK3 beta phosphorylation.
Curr.Biol. 11:44-49.
Table 1
Proteins concentrated at growing microtubule plus ends
in multi-cellular organisms
EB/RP family proteins
EB1
RP1
Cytoplasmic linker proteins and its binding proteins
CLIP -170
CLASPs (CLIP-associating proteins)
Dynein, dynactin complex and their binding proteins
Dynein
p150Glued
Dynamitin/p50
Arp1
LIS-1

 APC’s modular architecture led to attempts to identify
binding partners for each domain, and the discovery that APC binds
to b-catenin, a protein that functions in cell adhesion as well
as in Wnt-based signal transduction, provided the first significant
insight into the biological function of APC in molecular terms
(Rubinfeld et al., 1993; Su et al., 1993).
APC’s modular architecture led to attempts to identify
binding partners for each domain, and the discovery that APC binds
to b-catenin, a protein that functions in cell adhesion as well
as in Wnt-based signal transduction, provided the first significant
insight into the biological function of APC in molecular terms
(Rubinfeld et al., 1993; Su et al., 1993). 
