Multifunctional strands in tight junctions
Shoichiro Tsukita, Mikio Furuse and Masahiko Itoh
Department of Cell Biology, Kyoto University Faculty of
Medicine, Yoshida-Konoe, Sakyo-ku,
Kyoto 606-850, Japan.
Tight junctions are one mode of cell-to-cell adhesion in
epithelial and endothelial cellular sheets,ü@and are indispensable
for multicellular organisms. They act as a primary barrier to
the diffusion of solutes through the intercellular space, create
a boundary between the apical and the basolateral plasma membrane
domains, and recruit various cytoskeletal as well as signaling
molecules at their cytoplasmic surface. New insights into the
molecular architecture of tight junctions allow us now to discuss
the structure and functions of this unique cell-cell adhesion
apparatus in molecular terms.
The existence of separate fluid compartments with different molecular
compositions is of particular importance for the development and
maintenance of multicellular organisms. These compartments are
delineated by various cellular sheets, which function as barriers
to maintain the distinct internal environment of each compartment.
For example, renal tubules, blood vessels and the peritoneal cavity
are lined with epithelial, endothelial and mesothelial cellular
sheets, respectively. Within these sheets, individual cells are
mechanically linked with each other to maintain the structural
integrity of the sheet, and the intercellular space between adjacent
cells is sealed to prevent the diffusion of solutes through the
intercellular space. 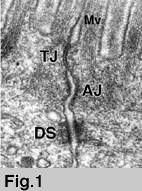
ü@ü@The junctional complex of simple epithelial cells is located
at the most-apical part of the lateral membrane and consists of
three distinct components: tight junctions (TJ), adherens junctions
and desmosomes (1 )(Fig.1). On ultra-thin section electron micrographs,
TJs appear as a series of apparent fusions ("kissing points"),
involving the outer leaflets of the plasma membranes of adjacent
cells (Fig.1, Fig.2b). At kissing points of TJs, the intercellular
space is completely obliterated, whereas in adherens junctions
and desmosomes, the apposing membranes are 15-20 nm apart (Fig.1b).
In simple epithelial cellular sheets, adherens junctions and desmosomes
mechanically link adjacent cells, whereas TJs are responsible
for intercellular sealing (2,3).
ü@ü@But many physiological situations require that various materials
are selectively transported across cellular sheets, and this occurs
either by transcellular transport through the cell or by paracellular
flux through TJs (4) (Box 1). So TJs
are not simply impermeable barriers: they show ion as well as
size selectivity, and vary in tightness depending on cell type
(3,5). 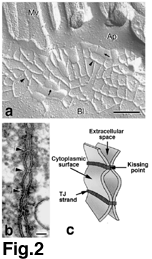
ü@ü@In addition to the "barrier function", TJs are thought
to be involved in a "fence function" (2,3). For the
vectorial transport of materials across cellular sheets, plasma
membranes are functionally divided into apical and basolateral
domains that face the luminal and serosal compartments, respectively.
Apical and basolateral membrane domains differ in the compositions
of integral membrane proteins as well as lipids. However, since
integral membrane proteins and lipids can diffuse laterally within
the plane of the lipid bilayer of plasma membranes, some diffusion
barrier is required at the border between apical and basolateral
membrane domains. Since TJs look like a fence within plasma membranes
at the most apical part of lateral membranes as shown below, it
has been suggested that TJs are the morphological counterpart
of a localized diffusion barrier.
ü@ü@In recent years, information on the molecular components of
TJs, and in particular their cell adhesion molecules, has accumulated.
Here, we will present an overview of our current understanding
of the structure and functions of TJs in molecular terms.
Ultrastructure and components of TJ strands
The morphology of TJs has been intensively analyzed by freeze-fracture
replica electron microscopy. On freeze-fracture replica electron
micrographs, TJs appear as a set of continuous, anastomosing intramembranous
particle strands or fibrils (TJ strands) on the P-face with complementary
vacant grooves on the E-face (6) (Fig.2a). The number of TJ strands
as well as the frequency of their ramification vary significantly
depending on cell type, producing marked variation in the morphology
of TJ strand networks. These observations led to our understanding
of the three-dimensional structure of TJs (Fig.2c). Each TJ strand
within the plasma membrane associates laterally with another TJ
strand in the apposing membrane of adjacent cells to form "paired"
TJ strands, where the intercellular space is obliterated. 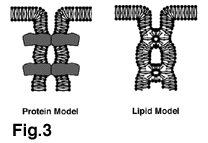
ü@ü@Two types of models have been proposed to explain the chemical
nature of TJ strands (Fig.3). In the 'protein model', TJ strands
represent units of integral membrane proteins polymerized linearly
within lipid bilayers, whereas in the 'lipid model' lipids organized
in inverted cylindrical micelles are proposed to constitute TJ
strands (7). Recent identification of TJ-specific integral membrane
proteins strongly supports the "protein" model, although
we cannot exclude the possibility that specific lipids may also
be important for the formation of TJ strands.
ü@ü@Occludin (~60 kDa) was identified as the first integral membrane
protein localized at TJs in chicken (8), and then also in mammals
(9). Occludin has four transmembrane domains, a long carboxyl-terminal
cytoplasmic domain and a short amino-terminal cytoplasmic domain
(Fig.4a). No occludin-related genes have been identified yet,
but two isoforms of occludin were found to be generated by alternative
splicing (10). 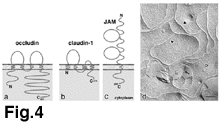
ü@ü@On immuno-replica electron microscopy, anti-occludin antibodies
exclusively labeled TJ strands (11), suggesting that occludin
is directly incorporated into TJ strands. Furthermore, as the
intensity of immunostaining with anti-occludin antibodies in various
tissues correlates well with the number of TJ strands, the density
of occludin molecules in TJ strands seems almost constant (11).
But TJ strands can also be formed without occludin, as in some
cell types such as endothelial cells in non-neuronal tissue and
in Sertoli cells (in the human testis), occludin was not detected
in TJ strands (12,13). More importantly, visceral endoderm cells
differentiated from occludin-deficient embryonic stem cells still
have well-developed networks of TJ strands (14). At present, the
physiological functions of occludin are not well understood. Its
possible functions will be discussed in detail below.
ü@ü@More recently, two other transmembrane protein "claudin-1
and claudin-2 " were identified as integral components of
TJ strands (15). ü@These proteins also possess four transmembrane
domains, but do not show any sequence similarity to occludin (Fig.4b).
To date, 24 members of the claudin family have been identified,
mainly through database searches, in mouse and human (16,17 )(Table 1).
There is accumulating evidence that claudins constitute the backbone
of TJ strands. Immuno-replica electron microscopy revealed that
claudins are exclusively localized on TJ strands (16,18,19). Exogenously
expressed claudins conferred cell-aggregation activity on L fibroblasts
with their concomitant concentration at cell-cell contact planes
(20) and led to the formation of a large network of TJ strand-like
structures (21) (Fig.4d). Occludin itself has no ability to reconstitute
such well-organized strands, but when occludin was introduced
into claudin-expressing L transfectants, it was incorporated into
reconstituted claudin-based strands (21).
ü@ü@The expression pattern of claudins varies considerably among
tissues (15,16) (Table 1). Some claudins
are known to be expressed in specific cell types; for example
claudin-5/TMVCF is expressed only in endothelial cells of blood
vessels (19), and claudin-11/OSP is only in oligodendrocytes and
Sertoli cells (18). Most cell types, however, express more than
two claudin species in various combinations to constitute TJ strands:
Within individual single strands, distinct species of claudins
are co-polymerized to constitute "heteropolymers", and
between adjacent strands within "paired" strands, claudins
adhere with each other in a homotypic as well as heterotypic manner
(22,23).
ü@ü@The last transmembrane component of TJs is JAM (junctional adhesion
molecule; ~40 kDa) (24). There are three JAM-related proteins
(25,26), which belong to the immunoglobulin superfamily: they
have a single transmembrane domain and their extracellular portion
is thought to be folded into two immunoglobulin-like domains (Fig.4c).
Preliminary freeze-fracture replica electron microscopy revealed
that exogenously expressed JAM does not reconstitute TJ strands
in L transfectants and that it associates laterally with the claudin-based
backbone of TJ strands in epithelial cells. JAM was shown to be
involved in cell-cell adhesion/junctional assembly of epithelial/endothelial
cells (24,25,27,28) as well as in the extravasation of monocytes
through endothelial cells (24), but our knowledge on its function
is still fragmentary.
A ziplock with diversified permeability
TJs vary in tightness in a tissue-dependent manner (2,3).
The tightness of TJs can be directly measured as transepithelial
electric resistance (TER). ü@The number of TJ strands was found
to correlate well with the TER values of TJs in various tissues
(29,30). For example, in the kidney, epithelial cells of the proximal
and distal tubules bear 1-2 and 4-7 TJ strands, respectively,
and the epithelial cells of the distal tubules exhibit much higher
TER than those of the proximal tubules. However, exceptions to
this correlation have also been reported (31,32). For example,
the two existing strains of Madin-Darby canine kidney (MDCK) epithelial
cells -MDCK I and MDCK II - show marked disparity in their TER.
Stevenson et al. reported that MDCK I cells have a 30-60 fold
higher TER than MDCK II cells, but the number of TJ strands in
these strains is very similar (33). These observations indicate
that individual paired TJ strands also vary in quality, i.e. tightness,
not only in number.
The number of strands. The number of TJ strands
is an important factor in determining the barrier properties of
TJs, but the molecular mechanism underlying regulation of the
strand number remains unknown. When MDCK I cells, which express
claudin-1 and claudin-4, were specifically depleted of claudin-4,
a marked decrease was observed in the number of TJ strands and
in their barrier function (34). Mice lacking claudin-11/OSP, which
is expressed specifically in oligodendrocytes and Sertoli cells
in wild-type mice, were recently generated, and in these mice
TJ strands were absent in myelin sheaths as well as in Sertoli
cells (35). Furthermore, when claudins were overexpressed in L
fibroblasts, a large network of TJ strands was formed (21). In
addition, overexpression of occludin in MDCK cells was also shown
to cause an increase in the number of TJ strands to some extent
(36). These findings would suggest that the number of TJ strands
is determined by the total amount of expressed claudins (and occludin)
in individual cells. However, the regulation of the number of
TJ strands is probably more complicated. In epithelial cells that
already express claudins, overexpression of claudins did not lead
to a significant increase in the number of TJ strands (37) , suggesting
that an upper limit exists. Interestingly, when a claudin-1 mutant
lacking its binding ability to underlying cytoskeletons were overexpressed
in MDCK cells, aberrant TJ strands were formed (37). This finding
suggests the possible involvement of the underlying proteins in
the regulation of the TJ strand number, but how the upper limit
is set remains a mystery.
ü@ü@In addition to the strand number, the complexity of the network
pattern may also be an important factor determining the barrier
properties of TJs (30). The network patterns of the reconstituted
TJs in L fibroblasts varied markedly among claudin species. For
example, claudin-1-induced TJ strands form a large network through
frequent ramifications (21), whereas claudin-11/OSP-induced strands
scarcely branched and ran parallel to each other (18). This observation
is likely to be relevant in vivo, as claudin-11/OSP-based
TJ strands in myelin sheaths and Sertoli cells are mostly parallel
with little branching (18,35). It is tempting to speculate that
the complexity of the TJ strand network is determined by the combination
and the mixing ratio of the expressed claudin species. 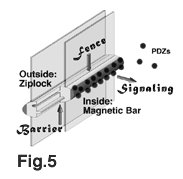
Extracellular aqueous pores. The extracellular
portion of TJ strands probably functions as a ziplock to create
a primary barrier against paracellular diffusion (Fig.5). By comparing
the TER and the morphology of TJ strands in various epithelia,
Claude found that as TJ strands increase in number, the TER value
increases logarithmically (30), and then to explain this relationship
the existence of aqueous pores was postulated within the paired
TJ strands, which take both open and closed states (5,30,38) (Box 2). As mentioned above, however, some
exceptions to this relationship between the number of TJ strands
and the TER value have been found (31,32). The difference in tightness
of individual TJ strands could be explained by the heterogeneity
of aqueous pores in terms of their probability being open or closed
(33).
ü@ü@But what is the chemical nature of these aqueous pores? Recent
studies on hereditary hypomagnesemia provided a clue to answer
this question (39). Most Mg++ is resorbed from the urine through
the paracellular pathway in the thick ascending limb of Henle,
but in these patients this resorption is reduced, resulting in
severe hypomagnesemia. Positional cloning identified claudin-16/paracellin-1
as the gene responsible for this disease. In good agreement, claudin-16/paracellin-1
is exclusively expressed in the thick ascending limb of Henle.
This finding suggested that claudin-16/paracellin-1 forms aqueous
pores that function as Mg++ "paracellular" channels.
The difference between MDCK I and MDCK II cells is probably also
due to their different expression of claudins (40): MDCK I express
primarily claudin-1 and claudin-4, whereas MDCK II cells also
express large amounts of claudin-2 in addition to claudin-1 and
-4. When claudin-2 was introduced into MDCK I cells, the TER value
of these MDCK I transfectants fell to the level of MDCK II cells
without any changes in the number of TJ strands. In contrast,
exogenously expressed claudin-3 did not affect the TER value of
MDCK I cells. Therefore, it is likely that claudin-2 constitutes
aqueous pores with high conductance within paired TJ strands of
MDCK II cells.
These findings led to the conclusion that claudins not only form
the backbone of TJ strands but also extracellular aqueous pores,
and that the combination and the mixing ratios of claudin species
determine the tightness of individual TJ strands (23). But it
is also possible that TJ strands are simply repeatedly broken
and annealed, and that this contributes to the tightness of individual
strands. To date, the information is not available regarding the
stability of strands, and this issue should be examined in detail
in molecular terms in future studies.
ü@ü@Occludin has also been shown to be involved in the barrier function
of TJs, but at present, how occludin is involved remains unclear.
Occludin-deficient mice were born normal, but as they grew up,
they began to show complex phenotypes including significant growth
retardation, chronic inflammation and hyperplasia of the gastric
epithelium and mineral deposition in the brain (41). TJs in most
organs of occludin-deficient mice such as intestinal epithelial
cells seem normal in terms of their morphology and TER. Consistently,
transfection of carboxyl-terminally truncated occludin into MDCK
cells induced redistribution of endogenous occludin, leaving occludin-deficient
TJ strands with normal appearance, but did not decrease their
TER (42). These findings are inconsistent with previous observation
that addition of synthetic peptides corresponding to the second
extracellular loop of occludin into culture medium removed endogenous
occludin from TJs, resulting in marked decrease in their TER value
(43). Interestingly, overexpression of full-length as well as
carboxyl-terminally truncated occludin in MDCK cells raised TER,
and, paradoxically, increased mannitol flux (36,37,42). These
observations suggested that occludin contributes to the electrical
barrier function of TJ to some extent and possibly to the formation
of aqueous pores within TJ strands through which non-charged solute
flux occurs. Although the paracellular flux of non-charged solute
has not yet been examined in occludin-deficient mice, it is possible
that the multiple defects found in these mice are attributed to
disappearance of putative "occludin-based" aqueous pores
from TJ strands. Of course, it is also possible that occludin
plays some important role in other TJ-related functions such as
fence function and/or signaling events rather than barrier function,
which could explain the cause of multiple defects in occludin-deficient
mice.
A magnetic bar for PDZ-containing proteins
The thickness of TJ strands (6) (~10 nm; see Fig.2a) is similar
to the diameter of the gap junctional channel (connexon) consisting
of 6 connexin molecules that also bear four transmembrane domains.
Therefore, it is not likely that claudins are aligned in a single
line to constitute TJ strands, but that they are packed more densely
in the strands. It is then expected that the cytoplasmic surface
of individual TJ strands appears as a tooth-brush consisting of
densely packed, numerous short carboxyl-terminal cytoplasmic tails
of claudins. In addition to these claudin tails, relatively long
carboxyl-terminal tails of occludin are probably intermingled.
ü@ü@Many cytosolic proteins have been reported to associate with
cytoplasmic surface of TJs. As the first component of TJs, a peripheral
membrane protein with a molecular mass of 220 kDa was identified
through monoclonal antibody production, and was named ZO-1 (Zonula
Occludens-1) (44). When ZO-1 was immunoprecipitated from cell
lysates of MDCK cells, two proteins with molecular masses of 160
kDa and 130 kDa were coprecipitated (45,46). As these proteins
were also localized at TJs, they were designated as ZO-2 and ZO-3,
respectively. ZO-1, ZO-2 and ZO-3 have sequence similarity with
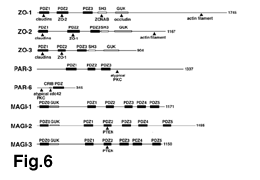
each other (47-50): they contain three PDZ domains (PDZ1, PDZ2
and PDZ3), one SH3 domain, and one guanylate kinase-like (GUK)
domain (Fig.6).
ü@ü@The PDZ domain was initially reported to specifically bind to
the carboxyl-terminal Glu-Ser/Thr-Asp-Val motif, but it is now
known that it recognizes more diverse 4-amino acid sequences most
ofthem ending in Val. Interestingly, most claudin tails have a
Val at their carboxyl termini (15,16); the only known exception
is claudin-12. This suggests that these carboxyl termini directly
bind to PDZ domains. If so, the cytoplasmic surface of TJ strands
may function as a magnetic bar that strongly attracts and recruits
many PDZ-containing proteins (Fig.5). Indeed, the PDZ1 domains
of ZO-1, ZO-2 and ZO-3 were recently shown to bind directly to
the carboxyl termini of claudins (51). No notable differences
were detected in the affinity of different claudins to PDZ1 domains.
ZO-1, ZO-2 and ZO-3 also directly bind to the carboxyl-terminal
tail of occludin, but their GUK domains, not PDZ domains, are
involved in this interaction (50,52-54). As ZO-1, ZO-2 and ZO-3
are localized to TJs in occludin-deficient mice, this interaction
may not be essential for their recruitment to TJs (41,51). Furthermore,
JAM, which is concentrated around TJ strands, also ends in Val,
and was recently shown to directly bind to ZO-1 (55,56) and other
PDZ-containing proteins (57). Thus, it is possible that JAM is
also involved in the recruitment of various PDZ-containing proteins
to TJs.
ü@ü@In addition to ZO-1, ZO-2 and ZO-3, several PDZ-containing proteins
are recruited to the cytoplasmic surface of TJs, but it remains
unknown whether these proteins directly bind to the carboxyl termini
of claudins (Fig.6): MAGI-1 (Membrane Associated Guanylate Kinase
Inverted-1) (58,59), MAGI-2 (60) and MAGI-3 (61), each of which
contains 6 PDZ domains, and mammalian homologues of C.elegans
PAR (partitioning) gene products, PAR-3 (ASIP, atypical PKC isotype-specific
interacting protein)62 and PAR-663-65, which contain three and
one PDZ domain(s), respectively. The list of PDZ-containing proteins
localized at TJs will probably continue to increase. These proteins
are multi-domain proteins, and may function as adapters at the
cytoplasmic surface of TJ strands, which recruit various proteins
including cytoskeletal as well as signaling molecules (Fig.6,
Table 2).
ü@ü@Through the recruitment of various types of proteins to TJs
via PDZ-containing proteins, a huge macromolecular complex is
expected to be formed at the cytoplasmic surface of TJ strands
(Fig.5). What is the physiological functions of this complex?
Firstly, as actin filaments bind to the carboxyl-terminal portions
of ZO-1/ZO-2 (53,54,66), this complex cross-links TJ strands to
actomyosin cytoskeletons. This interaction has been thought to
play a role in the regulation of TJ functions. Interestingly,
similar accumulation of PDZ-containing proteins occurs in the
postsynaptic density in neurons, where these PDZ-containing proteins
are directly involved in the synaptic signal transduction and
its regulation (67). In cell-matrix adhesion, a huge macromolecular
complex is known to be formed at integrin-based adhesion sites,
and to play crucial roles in the extracellular matrix-dependent
signaling, although this complex formation is not based on PDZ-containing
proteins (68). However, in cell-cell adhesion, such macromolecular
complex is not well developed at the cadherin-based adhesion site,
i.e. adherens junctions. Therefore, it is tempting to speculate
that the huge macromolecular complex formed at the cytoplasmic
surface of TJs plays central roles in the intercellular signaling
of epithelial/endothelial cells, being involved in the regulation
of their proliferation, differentiation and polarization. In this
connection, it is interesting to point out that TJs recruit a
tumor suppressor gene product (PTEN) (60,61), a quaternary complex
of cell polarity-related gene products (PAR-3, PAR-6, atypical
PKC, cdc42) (63-65) and vesicular transport-related proteins (Rab3B,
Rab13, Sec6/8 products) (69-71).
A fence within plasma membranes
TJ strands are heteropolymers of integral membrane proteins,
occludin and claudins, which are embedded within plasma membranes,
and continuously encircle the top of individual epithelial/endothelial
cells to delineate the border between the apical and basolateral
membrane domains. Therefore, it is likely that TJ strands act
as a 'fence', limiting the lateral diffusion of lipids and proteins
between the apical and basolateral membrane domains (Fig.5).
ü@ü@The apical membrane of epithelial cells is enriched in glycosphingolipids
and sphingomyelin (72,73). Interestingly, this membrane displays
a striking asymmetric organization of lipids across the lipid
bilayer, and glycosphingolipids as well as sphingomyelin are concentrated
in its outer leaflet (74,75). Such polarized localization of lipids
suggest the existence of the diffusion barrier, especially for
the lipids in the outer leaflet. This was confirmed experimentally.
When fluorescently labeled lipids were inserted into the outer
leaflet of the apical membrane of cultured epithelial cells, they
retained on the apical surface. In contrast, fluorescently labeled
lipids inserted into the inner leaflet of the apical membrane
quickly redistributed to the basolateral surface (76,77).
ü@ü@It is reasonable to speculate that TJs restrict the lateral
diffusion of not only lipids but also integral membrane proteins.
As the intercellular space is completely obliterated at TJs, integral
membrane proteins with extended extracellular portions could not
cross TJs. However, it is also clear that, in addition to TJs,
there are other mechanisms behind the asymmetric distribution
of certain integral membrane proteins within plasma membranes
(78): The cytoskeletal proteins underlying the plasma membranes
can restrict the lateral diffusion of proteins within membrane
domains and the targeted vesicle fusion is also important. Early
studies showed that the disruption of intercellular junctions
(for instance by incubation with low calcium medium) resulted
in the intermixture of membrane proteins from the apical and basolateral
domains (79,80). However, in these experiments, not only TJs but
also adherens junctions/desmosomes disappeared, and the cytoskeletal
architecture was also affected. Thus, it is difficult from these
experiments to conclude that TJs act as a diffusion barrier for
membrane proteins. This issue, i.e. the importance of TJs in the
asymmetric distribution of integral membrane proteins, remains
controversial (81). In this connection, expression of constitutively-active
RhoA and Rac1 small GTPases in MDCK cells was recently reported
to result in disorganization of TJ strand networks as well as
the disruption of the junctional fence for lipids but not for
integral membrane proteins (82).
ü@ü@When carboxyl-terminally truncated occludin was overexpressed
in cultured MDCK cells, fluorescently-labeled sphingomyelin added
to the apical membrane domain was redistributed to the basolateral
surface (42). The polarized distribution of integral membrane
proteins was not apparently affected. These findings suggest the
possible involvement of occludin in the diffusion barrier, especially
for lipids. The relationship between claudins and the diffusion
barrier in epithelial cells has not yet been examined. Considering
that occludin-deficient mice were born with normal epithelial
cells in the intestine and the kidney (41), it is still premature
to further discuss the relationship between the "fence"
function of TJs and occludin/claudins.
Future directions
TJs have attracted a great deal of interest from investigators
in various fields, but lack of information concerning the TJ-specific
integral membrane proteins has hampered studies of the molecular
biology of TJs. The recent identification of major components
of TJ strands have facilitated the molecular assessment of the
morphological and physiological observations of TJs that have
been accumulated over years. Based on the accumulated information
on occludin and claudins, we have discussed here multiple functions
of TJ strands at the molecular level; the barrier, signaling,
and fence functions (Fig.5). In addition to the issues described
above, the challenge of understanding many intriguing open questions
on TJs lies ahead of us.
ü@ü@The identification of occludin/claudins leads immediately to
many basic questions on the TJ strand itself. How are occludin
and heterogeneous claudins arranged in individual TJ strands?
To what extent are TJ strands dynamically polymerized and depolymerized?
How is it regulated? Are some lipids required for the polymerization
of occludin/claudins? How can TJ strands restrict the lateral
diffusion of lipids only in outer leaflets of the membranes? As
the polymerization of integral membrane proteins in a linear fashion
is unique, the elucidation of the basic physico-chemical properties
of TJ strands may constitute one of the big challenges for years
to come.
ü@ü@Several intriguing questions stand out at the cellular level.
One of the most pressing questions concerns the molecular mechanism
underlying the polarized formation of TJs at the most apical region
of the lateral membranes in epithelial cells. What is the relationship
between tight junctions, adherens junctions and desmosomes during
epithelial polarization? How are occludin, claudins, cadherins
and their underlying molecules integrated into the polarized junctional
complex during epithelial polarization? In this connection, it
should be pointed out that there is an alternative model for the
function of ZO-1/ZO-2/ZO-3 which was not discussed above. In that
model, ZO-1/ZO-2/ZO-3 recruit TJ proteins such as claudins and
occludin to their final destination at the interface between the
apical and basolateral membrane domains. Another outstanding issue
concerns the regulation of the TJ barrier. As indicated above,
TJs vary in tightness in a cell type-dependent manner. The tightness
of TJs is also known to be dynamically and finely regulated in
individual cells depending on various physiological and pathological
requirements (2,3) (see Box 1). The
information of the molecular mechanism underlying these regulations
is still fragmentary, but several signaling pathways such as serine/threonine
phosphorylation, tyrosine phosphorylation, heterotrimeric G proteins
and small G proteins are thought to be involved in their regulation
(83). The transcription of occludin was reported to be down-regulated
by TNF alpha/interferon gamma (84) and/or by activation of the
MAP kinase cascade (85,86), but there is no information available
regarding the transcriptional regulation of claudins by these
or other signaling pathways. The cytoplasmic tail of occludin
was shown to be heavily phosphorylated on serine and threonine
residues (87), whereas the phosphorylation of claudins has not
yet been examined.
ü@ü@Finally, another important challenge for future studies of TJs
is to examine their possible involvement in various diseases.
As mentioned above, mutations in claudin-16/paracellin-1 were
shown to cause hereditary hypomagnesemia (39). Furthermore, recent
positional cloning identified claudin-14 as the gene responsible
for hereditary deafness (88). This claudin species is expressed
in hair cells in the cochlea of the inner ear. As TJs in these
cells play crucial roles in establishment of two compositionally
distinct compartments in the inner ear, mutations in the claudin-14
gene would cause deafness. In addition to hereditary diseases,
claudins appear to have something to do with various pathological
conditions including inflammation (89). Furthermore, the involvement
of occludin86 as well as claudins90 in tumorigenesis has been
suggested in recent years.
ü@ü@We are only just beginning to understand the functions of TJs
in molecular terms. Our picture of the molecular architecture
of TJs remains incomplete, and other important constituents need
to be identified. Further development of the molecular biology
of TJs will lead to a better understanding of the roles of TJs
not only in normal physiology but also in disease.
References
1. Farquhar, M. G. & Palade, G. E. Junctional complexes
in various epithelia. J. Cell Biol. 17, 375-412
(1963).
2. Schneeberger, E. E. & Lynch, R. D. Structure, function,
and regulation of cellular tight junctions. Am.J. Physiol.
262, L647-L661 (1992).
3. Gumbiner, B. Breaking through the tight junction barrier. J.Cell
Biol. 123, 1631-1633 (1993).
4. Spring, K. Routes and mechanism of fluid transport by epithelia.
Annu. Rev. Physiol. 60, 105-119 (1998).
5. Reuss, L. Tight junction permeability to ions and water. In
ęTight Junctionsė (Cereijido,M. ed.) p.49-66, CRC press, London.
(1992).
6. Staehelin, L. A. Further observations on the fine structure
of freeze-cleaved tight junctions. J.Cell Sci. 13,
763-786 (1973).
7. Kachar, B. & Reese, T. S. Evidence for the lipidic nature
of tight junction strands. Nature 296, 464-466 (1982).
8. Furuse, M. et al. Occludin: a novel integral membrane protein
localizing at tight junctions. J.Cell Biol. 123,
1777-1788 (1993).
9. Ando-Akatsuka,Y. et al. Interspecies diversity of the occludin
sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo
homologues. J.Cell Biol. 133, 43-47 (1996).
10. Muresan, Z., Paul, D. L. & Goodenough, D. A. Occludin1B,
a variant of the tight junction protein. Mol.Biol.Cell 11,
627-634 (2000).
11. Saitou, M. et al. Mammalian occludin in epithelial cells:
its expression and subcellular distribution. Eur.J.Cell Biol.
73, 222-231 (1997).
12. Hirase, T. et al. Occludin as a possible determinant of tight
junction permeability in endothelial cells. J.Cell Sci. 110,
1603-1613 (1997).
13. Moroi, S. et al. Occludin is concentrated at tight junctions
of mouse/rat but not human/guinea pig Sertoli cells in testes.
Am.J.Physiol. 274, C1708-C1717 (1998).
14. Saitou, M. et al. Occludin-deficient embryonic stem cells
can differentiate into polarized epithelial cells bearing tight
junctions. J.Cell Biol. 141, 397-408 (1998).
15. Furuse, M., Fujita, K., Hiiragi, T., Fujimoto, K. & Tsukita,
Sh. Claudin-1 and -2: Novel integral membrane proteins localizing
at tight junctions with no sequence similarity to occludin. J.Cell
Biol. 141, 1539-1550 (1998).
16. Morita, K., Furuse, M., Fujimoto, K. & Tsukita, Sh. Claudin
multigene family encoding four-transmembrane domain protein components
of tight junction strands. Proc.Natl.Acad.Sci.USA 96,
511-516 (1999).
17. Tsukita, Sh. & Furuse, M. Occludin and claudins in tight
junction strands: Leading or supporting players? Trend.Cell
Biol. 9, 268-273 (1999).
18. Morita, K., Sasaki, H., Fujimoto, K., Furuse, M., & Tsukita,
Sh.. Claudin-11/OSP-based tight junctions in myelinated sheaths
of oligodendrocytes and Sertoli cells in testis. J.Cell Biol.
145, 579-588 (1999).
19. Morita, K., Sasaki, H., Furuse, M. & Tsukita, Sh. Endothelial
claudin: Claudin-5/TMVCF constitutes tight junction strands in
endothelial cells. J.Cell Biol. 147, 185-194 (1999).
20. Kubota, K. et al. Ca++-independent cell adhesion activity
of claudins, integral membrane proteins of tight junctions. Curr.Biol.
9, 1035-1038 (1999).
21. Furuse, M., Sasaki, H. Fujimoto, K. & Tsukita, Sh. A single
gene product, claudin-1 or -2, reconstitutes tight junction strands
and recruits occludin in fibroblasts. J.Cell Biol. 143,
391-401 (1998).
22. Furuse, M., Sasaki, H. & Tsukita, Sh. Manner of interaction
of heterogeneous claudin species within and between tight junction
strands. J.Cell Biol. 147, 891-903 (1999).
23. Tsukita, Sh. & Furuse, M. Pores in the wall: Claudins
constitute tight junction strands containing aqueous pores. J.Cell
Biol. 149, 13-16 (2000).
24. Martin-Padura, I. et al. Junctional adhesion molecule, a novel
member of the immunoglobulin superfamily that distributes at intercellular
junctions and modulates monocyte transmigration. J.Cell Biol.
142, 117-127 (1998).
25. Palmeri, D., van Zante, A., Huang, C. C., Hemmerich, S. &
Rosen, S.D. Vascular endothelial junction-associated molecule,
a novel member of the immunoglobulin superfamily, is localized
to intercellular boundaries of endothelial cells. J.Biol Chem.
275, 19139-19145 (2000).
26. Aurrand-Lons, M. A., Duncn, L., Du Pasquire, L. & Imhof,
B. A. Cloning of JAM-2 and JAM-3; an emerging junctional adhesion
molecular family? Curr.Top.Microbiol.Immunol. 251,
91-98 (2000).
27. Bazzoni, G. et al. Homophilic interaction of junctional adhesion
molecule. J.Biol.Chem. 275, 30970-30976 (2000).
28. Liu, Y., Nustrat, A., Schnell, F. J., Reaves, T. A., Walsh,
S., Pochet, M., and Parkos, C. A. Human junction adhesion molecule
regulates tight junction resealing in epithelia. J.Cell Sci.
113, 236 3-23764 (2000).
29. Claude, P. & Goodenough, D. A. Fracture faces of zonulae
occludentes from ętightė and ęleakyė epithelia. J.Cell Biol.
58, 390-400 (1973).
30. Claude, P. Morphological factors influencing transepithelial
permeability: a model for the resistance of the zonula occludens.
J.Membr.Biol. 10, 219-232 (1978).
31. Martinez-Palomo, A. & Erlij, D. Structure of tight junctions
in epithelia with different permeability. Proc.Natl.Acd.Sci.USA.
72, 4487-4491 (1975).
32. Mollgard, K., Malinowski, D. N. & Saunders, N. R. Lack
of correlation between tight junction morphology and permeability
properties in developing choroid plexus. Nature 264,
293-294 (1976).
33. Stevenson, B. R., Anderson, J. M., Goodenough, D. A. &
Mooseker, M. S. Tight junction structure and ZO-1 content are
identical in two strains of Madin-Darby Canine Kidney cells which
differ in transepithelial resistance. J.Cell Biol. 107,
2401-2408 (1988).
34. Sonoda, N. et al. Clostridium perfringens enterotoxin
fragment removes specific claudins from tight junction strands:
Evidence for direct involvement of claudins in tight junction
barrier. J.Cell Biol. 147, 195-204 (1999).
35. Gow, A. et al. CNS myelin and Sertoli cell tight junction
strands are absent in OSP/claudin-11 null mice. Cell 99,
649-659 (1999).
36. McCarthy, K. M. et al. Occludin is a functional component
of the tight junction. J.Cell Sci. 109, 2287-2298
(1996).
37. McCarthy, K. M. et al. Inducible expression of claudin-1-myc
but not occludin-VSV-G results in aberrant tight junction strand
formation in MDCK cells. J.Cell Sci. 113, 3387-3398 (2000).
38. Cereijido, M., Gonzales-Mariscal, L. & Contreras, G. Tight
junction: barrier between higher organisms and environment. NIPS
4, 72 (1989).
39. Simon, D. B. et al. Paracellin-1, a renal tight junction protein
required for paracellular Mg2+ resorption. Science 285,
103-106 (1999).
40. Furuse, M., Furuse, K., Sasaki, H. & Tsukita, Sh. Conversion
of Zonula Occludentes from tight to leaky strand type by
introducing claudin-2 into MDCK I cells. J.Cell Biol. in
press (2001).
41. Saitou, M. et al. Complex phenotype of mice lacking occludin,
a component of tight junction strands. Mol.Biol.Cell 11,
4131-4142 (2000).
42. Balda, M. S. et al. Functional dissociation of paracellular
permeability and transepithelial electrical resistance and disruption
of the apical-basolateral intramembrane diffusion barrier by expression
of a mutant tight junction membrane protein. J.Cell Biol. 134,
1031-1049 (1996).
43. Wong, V. & Gumbiner, B. M.. A synthetic peptide corresponding
to the extracellular domain of occludin perturbs the tight junction
permeability barrier. J.Cell Biol. 136, 399-409
(1997).
44. Stevenson, B. R., Siliciano, J. D., Mooseker, M. S. &
Goodenough, D. A. Identification of ZO-1: a high molecular weight
polypeptide associated with the tight junction (zonula occludens)
in a variety of epithelia. J.Cell Biol. 103, 755-766
(1986).
45. Gumbiner, B., Lowenkopf, T. & Apatira, D. Identification
of a 160-kDa polypeptide that binds to the tight junction protein
ZO-1. Proc.Natl.Acad.Sci.USA. 88, 3460-3464 (1991).
46. Balda, M. S., Gonzćlez-Mariscal, L., Matter, K., Cereijido,
M. & Anderson, J. M. Assembly of the tight junction: The role
of diacylglycerol. J.Cell Biol. 123, 293-302 (1993).
47. Itoh, M. et al. The 220-kD protein colocalizing with cadherins
in non-epithelial cells is identical to ZO-1, a tight junction-associated
protein in epithelial cells: cDNA cloning and immunoelectron microscopy.
J.Cell Biol. 121, 491-502 (1993).
48. Willott, E., Balda, M. S., Fanning, A. S., Jameson, B., van
Itallie, C. & Anderson, J.M. The tight junction protein ZO-1
is homologous to the Drosophila discs-large tumor suppresser
protein of septate junctions. Proc.Natl.Acad.Sci.USA. 90,
7834-7831 (1993).
49. Jesaitis, L. A. & Goodenough, D. A. Molecular characterization
and tissue distribution of ZO-2, a tight junction protein homologous
to ZO-1 and the Drosophila discs-large tumor suppresser protein.
J. Cell Biol. 124, 949-961 (1994).
50. Haskins, J., Gu, L., Wittchen, E. S., Hibbard, J. & Stevenson,
B. R. ZO-3, a novel member of the MAGUK protein family found at
the tight junction, interacts with ZO-1 and occludin. J.Cell
Biol. 141, 199-208 (1998).
51. Itoh, M., Furuse, M., Morita, K., Kubota, K,, Saitou. M. &
Tsukita Sh. Direct binding of three tight junction-associated
MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins.
J.Cell Biol. 147, 1351-1367 (1999)
52. Furuse, M. et al. Direct association of occludin with ZO-1
and its possible involvement in the localization of occludin at
tight junctions. J.Cell Biol. 127, 1617-1626 (1994).
53. Itoh. M, Morita, K. & Tsukita, Sh. Characterization of
ZO-2 as a MAGUK family member associated with tight and adherens
junctions with a binding affinity to occludin and a catenin. J.Biol.Chem.
274, 5981-5986 (1999).
54. Fanning, A. S., Jameson, B. J., Jesaitis, L. A. & Anderson,
J. M. The tight junction protein ZO-1 establishes a link between
the transmembrane protein occludin and the actin cytoskeleton.
J.Biol.Chem. 273, 29745-29753 (1998).
55. Bazzoni, G., Martinez-Estrada, O.M., Orsenigo, F., Cordenonsi,
M., Citi, S. & Dejana, E. Interaction of Junctional Adhesion
Molecule with the tight junction components ZO-1, cingulin, and
occludin. J. Biol. Chem. 275, 20520-20526 (2000).
56. Ebnet, K., Schulz, C. U., Meyer Zu Brickwedde, M. K., Pendle,
G. G. & Vestweber, D. Junctional adhesion molecule interacts
with the PDZ domain-containing proteins AF-6 and ZO-1. J.Biol.Chem.
275, 27979-27988 (2000).
57. Martinez-Estrada, O. M., Villa, A., Breviario, F., Orsenigo,
F., Dejana, E. & Bazzoni, G. Association of junctional adhesion
molecule with calcium/calmodulin-dependent serine protein kinase
(CASK/LIN-2) in human epithelial Caco-2 cells. J.Biol.Chem.
in press (2000).
58. Dobrosotskaya, I., Guy, R. K. & James, G. L. MAGI-1, a
membrane-associated guanylate kinase with a unique arrangement
of protein-protein interaction domains. J.Biol.Chem. 272,
31589-31597 (1997).
59. Ide, N. et al. Localization of membrane-associated guanylate
kinase (MAGI)-1/BAI-associated protein (BAP) 1 at tight junctions
of epithelial cells. Oncogene 18, 7810-7815 (1999).
60. Wu, X. et al. Evidence for regulation of the PTEN tumor suppressor
by a membrane-localized multi-PDZ domain containing scaffold protein
MAGI-2. Proc.Natl.Acad.Sci.USA. 97, 4233-4238 (2000).
61. Wu, X. et al. Interaction of the tumor suppressor PTEN/MMAC
with a PDZ domain of MAGI3, a novel membrane-associated guanylate
kinase. J.Biol.Chem. 275, 21477-21485 (2000).
62. Izumi, Y. et al. An atypical PKC directly associates and colocalizes
at the epithelial tight junction with ASIP, a mammalian homologue
of Caenorhabditis elegans polarity protein PAR-3. J.Cell
Biol. 143, 95-106 (1998).
63. Joberty, G., Petersen, C., Gao, L. & Macara, I. G. The
cell-polarity protein Par6 links Par3 and atypical protein kinase
C to Cdc42. Nature Cell Biol. 2, 531-539 (2000)
64. Lin, D., Edwards, A. S., Fawcett, J. P., Mbamalu, G., Scott,
J. D. & Pawson, T. A mammalian PAR-3-PAR-6 complex implicated
in Cdc42/Rac1 and aPKC signalling and cell polarity. Nature
Cell Biol. 2, 540-547 (2000)
65. Qiu, R.G., Abo, A. & Steven Martin, G. A human homolog
of the C. elegans polarity determinant par-6 links rac
and cdc42 to PKCzeta signaling and cell transformation. Curr.
Biol. 10, 697-707 (2000)
66. Itoh, M., Nagafuchi, A., Moroi, S. & Tsukita, Sh. Involvement
of ZO-1 in cadherin-based cell adhesion through its direct binding
to a catenin and actin filaments. J.Cell Biol. 138,
181-192 (1997).
67. Hata, Y., Nakanishi, H. & Takai, Y. Synaptic PDZ domain-containing
proteins. Neurosci.Res. 32, 1-7 (1998).
68. Giancotti, F.G. & Ruoslahti, E. Integrin signaling. Science
285, 1028-1032 (1999).
69. Weber, E. et al. Expression and polarized targeting of a rab3
isoform in epithelial cells. J.Cell Biol. 125, 583-594
(1994).
70. Zahraoui, A. et al. A small rab GTPase is distributed in cytoplasmic
vesicles in non polarized cells but colocalizes with the tight
junction marker ZO-1 in polarized epithelial cells. J.Cell
Biol. 124, 101-115 (1994).
71. Grindstaff, K. K. et al. Sec6/8 complex is recruited to cell-cell
contacts and specifies transport vesicle delivery to the
basal-lateral membrane in epithelial cells. Cell 93,
731-740 (1998).
72. Forstner, G.G. & Wherrett, J.R. Plasma membrane and mucosal
glycosphingolipids in the rat intestine. Biochim.Biophys.Acta
306, 446-459 (1973).
73. Chapelle, S. & Gilles-Baillien, M. Phospholipids and cholesterol
in brush border and basolateral membranes from rat intestinal
mucosa. Biochim.Biophys.Acta 753, 269-271 (1983).
74. Barsukov, L.I., Bergelson, L.D., Spiess, M., Hauser, H. &
Semenza, G. Phospholipid topology and flip-flop in intestinal
brush-border membrane. Biochim.Biophys.Acta 862,
87-99 (1986).
75. Rothman, J.E., Tsai, D.K., Dawidowicz, E.A. & Lenard,
J. Transbilayer phospholipid asymmetry and its maintenance in
the membrane of influenza virus. Biochemistry 15,
2361-2370 (1976).
76. Dragsten, P.R., Blumenthal, R. & Handler, J.S. Membrane
asymmetry in epithelia: is the tight junction a barrier to diffusion
in the plasma membrane? Nature 294,718-722 (1981).
77. van Meer, G. & Simon, K. The function of tight junctions
in maintaining differences in lipid composition between the apical
and basolateral cell surface domains of MDCK cells. EMBO J.
5, 1455-1464.
78. Nelson, W.J. Regulation of cell surface polarity from bacteria
to mammals. Science 258, 948-954 (1992).
79. Pisam, M. & Ripoche, P. Redistribution of surface macromolecules
in dissociated epithelial cells. J.Cell Biol. 71,
909-920 (1976).
80. Ziomek, C.A., Shulman, S. & Edidin, M. Redistribution
of membrane proteins in isolated mouse intestinal epithelial cell.
J.Cell Biol. 86, 849-857 (1980).
81. Vega-Salas, D.E., Salas, P.J.I., Gundersen, D. & Rodriguez-Boulan,
E. Formation of the apical pole of epithelial (Madin-Darby canine
kidney) cells: polarity of an apical protein is independent of
tight junctions while segregation of a basolateral marker requires
cell-cell interaction. J.Cell Biol. 104, 905-916
(1987).
82. Jou, T-S., Schneeberger, E. E. & Nelson, W.J. Structural
and functional regulation of tight junctions by RhoA and Rac1
small GTPases. J.Cell Biol. 142, 101-115 (1998).
83. Tsukita, Sh., Furuse, M. & Itoh, M. Structural and signaling
molecules come together at tight junctions. Curr.Opin.Cell
Biol. 11, 628-633 (1999).
84. Mankertz, J. et al. Expression from the human occludin promoter
is affected by tumor necrosis factor a and interferon g. J.Cell
Sci. 113, 2085-2090 (2000).
85. Chen, Yh., Lu, Q., Schneeberger, E. E. & Goodenough, D.
A. Restoration of tight junction structure and barrier function
by down-regulation of the mitogen-activated protein kinase pathway
in ras-transformed Madin-Darby canine kidney cells. Mol.Biol.Cell
11, 849-862 (2000).
86. Li, D. & Mrsny, R. J. Oncogenic Raf-1 disrupts epithelial
tight junctions via downregulation of occludin. J.Cell Biol.
148, 791-800 (2000).
87. Sakakibara, A., Furuse, M., Saitou, M., Ando-Akatsuka, Y.
& Tsukita, Sh.. Possible involvement of phosphorylation of
occludin in tight junction formation. J.Cell Biol. 137,
1393-1401 (1997).
88. Wilcox, E. R. et al. Mutations in the gene encoding tight
junction claudin-14 cause autosomal recessive deafness DFNB29.
Cell 104, 165-172 (2001).
89. Kinugasa, T., Sakaguchi, T. & Reinecker, H. C. Claudins
regulate the intestinal barrier in response to immune mediators.
Gastroenterology 118, 1001-1011 (2000).
90. Hough, C. D. et al. Large-scale serial analysis of gene expression
reveals genes differentially expressed in ovarian cancer. Cancer
Res. 60, 6281-6267 (2000).
91. Madara, J. L. Tight junction dynamics: Is paracellular transport
regulated? Cell 53, 497-498 (1988).
92. Powell, D. W. Barrier function of epithelia. Am.J.Physiol.
241, G275-G288 (1981).
93. Madara, J. L. Regulation of the movement of solutes across
tight junctions. Annu.Rev.Physiol. 60, 143-159 (1998).
94. Briehl, M.M. & Miesfeld, R.L. Isolation and characterization
of transcripts induced by androgen withdrawal and apoptotic cell
death in the rat ventral prostate. Mol.Endocrinol. 5,
1381-1388 (1991).
95. Katahira, J., Inoue, N., Horiguchi, Y., Matsuda, M. &
Sugimoto, N. Molecular cloning and functional characterization
of the receptor for Clostridium perfringens enterotoxin.
J.Cell Biol. 136, 1239-1247 (1997).
96. Sirotkin, H. et al. Identification, characterization, and
precise mapping of a human gene encoding a novel membrane-spanning
protein from the 22q11 region deleted in Velo-Cardio-Facial syndrome.
Genomics 42, 245-251 (1997).
97. Bronstein, J.M., Popper, P., Micevych, P.E. & Farber,
D.B. Isolation and characterization of a novel oligodendrocyte-specific
protein. Neurology 47, 772-778 (1996).
98. Citi, S., Sabanay, H., Jakes, R., Geiger, B. & Kendrick-Jones,
J. Cingulin, a new peripheral component of tight junctions. Nature
333, 272-276 (1988).
99. Cordenonsi, M. et al. Cingulin contains globular and coiled-coil
domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J.Cell
Biol. 147, 1569-1582 (1999).
100. Zhong, Y. et al. Monoclonal antibody 7H6 reacts with a novel
tight junction-associated protein distinct from ZO-1, cingulin,
and ZO-2. J.Cell Biol. 120, 477-483 (1993).
101. Keon, B. H., SchŖfer, S., Kuhn, C., Grund, C. & Franke,
W. W. Symplekin, a novel type of tight junction plaque protein.
J.Cell Biol. 134, 1003-1018 (1996).
102. Saha, C., Nigam, S. K. & Denker, B. M. Involvement
of Gai2 in the maintenance and biogenesis of epithelial cell tight
junctions. J.Biol.Chem. 273, 21629-21633 (1998).
103. Balda, M. S. & Matter, K. The tight junction protein
ZO-1 and an interacting transcription factor regulate ErbB-2 expression.
EMBO J. 19, 2024-2033 (2000).
104. Nakamura, T. et al. huASH1 protein, a putative transcription
factor encoded by a human homologue of the Drosophila ash1
gene, localizes to both nuclei and cell-cell tight junctions.
Proc.Natl.Acad.Sci.USA 97, 7284-7289 (2000).






