About Us
Membrane proteins, including G protein-coupled receptors (GPCRs), transporters, ion channels, and integral membrane enzymes are involved in a large variety of pivotal biological functions and currently the most important class of therapeutic targets. Despite substantial interest in these targets, structure-based drug design (SBDD) has been challenging due to a paucity of high-resolution structural information. This is reflected in the difficulties encountered with the production, stability, and crystallization of membrane proteins. To overcome the bottlenecks, our laboratory has developed a state-of-the-art technical platform for membrane protein crystallography and has been studying structures of “druggable” membrane protein targets. The crystal structures will open up excellent opportunities in structure-based ligand discovery and/or SBDD. Research is being conducted in cooperation with the Membrane Protein Laboratory (MPL), a joint venture between the British synchrotron radiation facility Diamond Light Source and Imperial College London, as well as an X-ray free electron laser facility, SACLA, in Japan. Our laboratory is ideal for postdoctoral fellows and graduate students who aspire to build up an international career.
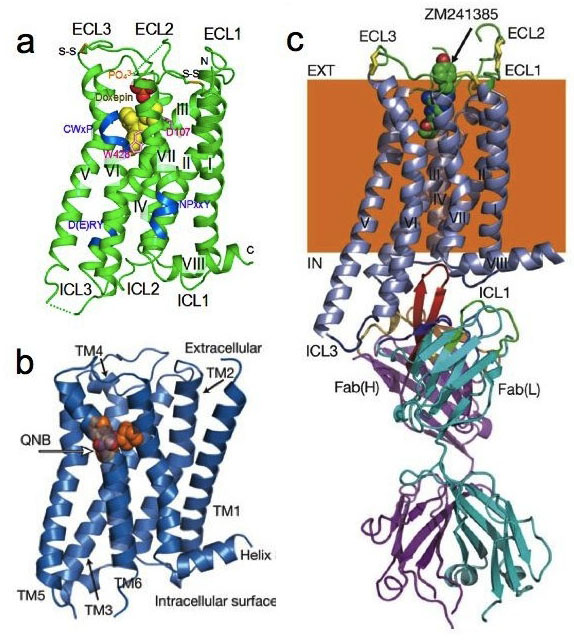
X-ray crystallography of human G protein-coupled receptors (GPCRs).
- (a) Histamine H1 receptor (H1R) complexed with doxepin, a first-generation H1R antagonist.
- (b) M2 muscarinic acetylcholine receptor complexed with QNB, an antagonist.
- (c) A2a adenosine receptor complexed with an antagonist, ZM241385, and an inverse agonist antibody fragment.
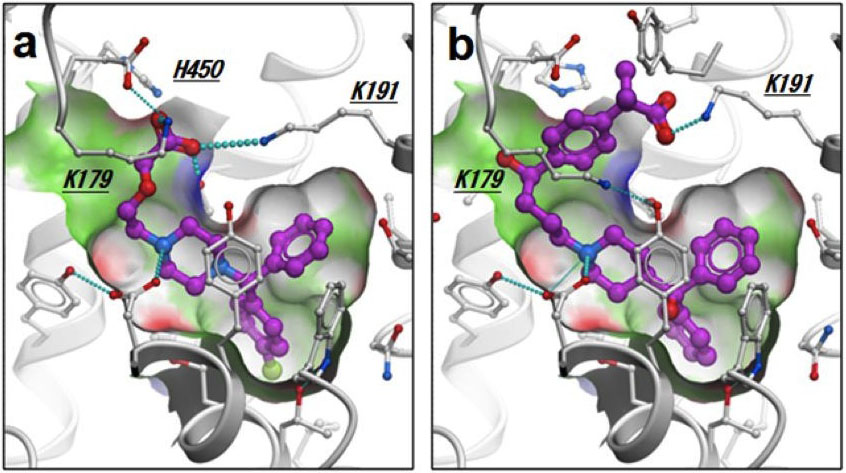
In silico docking simulations of second-generation selective H1R antagonists with the H1R ligand-binding pocket.
- (a) A model of H1R complexed with levocetirizine.
- (b) A model of H1R complexed with fexofenadine.
Recent Selected Publications
- New!
Shiimura Y*#, Im D#, Tany R#, Asada H, Kise R, Kurumiya E, Masuho HW, Yasuda S, Matsui K, Kishikawa J, Kato T, Murata T, Kojima M, Iwata S* & Masuho I* (#Contributed equally) (2025) - “The structure and function of the ghrelin receptor coding for drug actions”
- Nat Struct Mol Biol.
- DOI:10.1038/s41594-024-01481-6.
- Related PDB Entries:8JSR
*Im D, Jormakka M, Juge N, Kishikawa J, Kato T, Sugita Y, Noda T, Uemura T, Shiimura Y, Miyaji T, Asada H, *Iwata S. (2024)- Neurotransmitter recognition by human vesicular monoamine transporter 2
- Nat Commun.
- DOI:DOI: 10.1038/s41467-024-51960-z
- Related PDB Entries:8WRD 8WRE 8WVG
Im D, Kishikawa JI, Shiimura Y, Hisano H, Ito A, Fujita-Fujiharu Y, Sugita Y, Noda T, *Kato T, *Asada H. and *Iwata S. (2023)- Structural insights into the agonists binding and receptor selectivity of human histamine H4 receptor
- Nat Commun.
- DOI:DOI: 10.1038/s41467-023-42260-z
- Related PDB Entries:7YFC 7YFD
Asami J# , Terakado-Kimura K# , Fujita-Fujiharu Y# , Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, *Nomura N and *Ohto U. (#co-first authors) (2022)- Structure of bile acid transporter NTCP crucial for hepatitis B virus entry
- Nature
- DOI:10.1038/s41586-022-04845-4
- Related PDB Entries:
Park JH, Iwamoto M, Yun JH, Uchikubo-Kamo T, Son D, Jin Z, Yoshida H, Ohki M, Ishimoto N, Mizutani K, Oshima M, Muramatsu M, Wakita T, Shirouzu M, Liu K, Uemura T, Nomura N, Iwata S, Watashi K, Tame JRH, Nishizawa T, *Lee W and * Park SY. (2022)- Structural insights into the HBV receptor and bile acid transporter NTCP
- Nature
- DOI:10.1038/s41586-022-04857-0
- Related PDB Entries:7FCI
Kishi K, Kim YS, Fukuda M, Inoue M, Kusakizako T, Wang PY, Ramakrishnan C, Byrne EFX, Thadhani E, Paggi JM, Matsui T, Yamashita K, Nagata T, Konno M, Quirin S, Lo M, Benster T, Uemura T, Liu K, Shibata M, Nomura N, Iwata S, Nureki O, Dror RO, Inoue K, *Deisseroth K and *Kato H. (2022)- Structural basis for channel conduction in the pump-like channelrhodopsin ChRmine
- Cell 185:1-18
- DOI:10.1016/j.cell.2022.01.007
- Related PDB Entries:7W9W
Neville SL, Sjohamn J, Watts JA, MacDermott-Opeskin H, Fairweather SJ, Ganio K, Carey Hulyer A, McGrath AP, Hayes AJ, Malcolm TR, Davies MR, Nomura N, Iwata S, O'Mara ML, *Maher MJ and *McDevitt CA. (2021)- The structural basis of bacterial manganese import
- Science Advance 7(32); eabg3980
- DOI:10.1126/sciadv.abg3980 PubMed ID: 34362732
- Related PDB Entries:7KYO.7KYP
Ghilarov D, Inaba-Inoue S, Stepien P, Qu F, Michalczyk E, Pakosz Z, Nomura N, Ogasawara S, Walker GC, Rebuffat S, Iwata S, *Heddle JG and *Beis K. (2021)- Molecular mechanism of SbmA, a promiscuous transporter exploited by antimicrobial peptides
- Science Advances 7(37): eabj5363
- DOI:10.1126/sciadv.abj5363
- Related PDB Entries:7P34
- Our paper selected for online cover!

Okamoto H.H, Miyauchi H, Inoue A, Raimondi F, Tsujimoto H, Kusakizako T, Shihoya W, Yamashita K, Suno R, Nomura N, Kobayashi T, Iwata S, Nishizawa T and Nureki O.(2021)- Cryo-EM structure of the human MT1-Gi signaling complex
- Nat Struct Mol Biol. Online
- DOI:10.1038/s41594-021-00634-1
- Related PDB Entries:7DB6
Oda K, Nomura T, Nakane T, Yamashita K, Inoue K, Ito S, Vierock J, Hirata K, Maturana AD, Katayama K, Ikuta T, Ishigami I, Izume T, Umeda R, Eguma R, Oishi S, Kasuya G, Kato T, Kusakizako T, Shihoya W, Shimada H, Takatsuji T, Takemoto M, Taniguchi R, Tomita A, Nakamura R, Fukuda M, Miyauchi H, Lee Y, Nango E, Tanaka R, Tanaka T, Sugahara M, Kimura T, Shimamura T, Fujiwara T, Yamanaka Y, Owada S, Joti Y, Tono K, Ishitani R, Hayashi S, Kandori H, Hegemann P, Iwata S, Kubo M, Nishizawa T and Nureki O.(2021)- Time-resolved serial femtosecond crystallography reveals early structural changes in channelrhodopsin
- eLife. 10:e62389.
- DOI:10.7554/eLife.62389. PubMed ID: 33752801
- Related PDB Entries:7C86 (dark),7E6Y (1 ms),7E6Z (50 ms),7E70 (250 ms),7E71 (1 ms),7E6X(4ms)
Maeda S, Shiimura Y, Asada H, Hirata K, Luo F, Nango E, Tanaka N, Toyomoto M, Inoue A, Aoki J, *Iwata S and *Hagiwara M.(2021)- Endogenous agonist-bound S1PR3 structure reveals determinants of G protein-subtype bias
- Science Adv. 7: eabf5325
- DOI:10.1126/sciadv.abf5325
- Related PDB Entries:7C4S
Im D, Inoue A, Fujiwara T, Nakane T, Yamanaka Y, Uemura T, Mori C, Shiimura Y, Kimura KT, Asada H, Nomura N, Tanaka T, Yamashita A, Nango E, Tono K, Kadji FMN, Aoki J, Iwata S and Shimamura T.- Structure of the dopamine D2 receptor in complex with the antipsychotic drug spiperone.
- Nat Commun.11(1):6442 (2020)
- DOI:10.1038/s41467-020-20221-0. PubMed ID: 33353947
- Related PDB Entries:7DFP
Nojima S, Fujita Y, Kimura KT, Nomura N, Suno R, Morimoto K, Yamamoto M, Noda T, Iwata S, Shigematsu H and Kobayashi T. (2020)- Cryo-EM Structure of the Prostaglandin E Receptor EP4 Coupled to G Protein Structure.
- Structure.29(3):252-260 (2020)
- DOI:10.1016/j.str.2020.11.007. PubMed ID: 33264604
- Related PDB Entries:7D7M
Shiimura Y, Horita S, Hamamoto A, Asada H, Hirata K, Tanaka M, Mori K, Uemura T, Kobayashi T, Iwata S and Kojima M. (2020)- Structure of an antagonist-bound ghrelin receptor reveals possible ghrelin recognition mode.
- Nat Commun. 11:1-9
- DOI:10.1038/s41467-020-17554-1.
- Related PDB Entries:6KO5,6KS2
Asada H, Inoue A, Ngako Kadji FM, Hirata K, Shiimura Y, Im D, Shimamura T, Nomura N, Iwanari H, Hamakubo T, Kusano-Arai O, Hisano H, Uemura T, Suno C, Aoki J and Iwata S.(2019)- The Crystal Structure of Angiotensin II Type 2 Receptor with Endogenous Peptide Hormone.
- Structure.28: 1-8
- DOI:10.1016/j.str.2019.12.003. PubMed ID: 31899086
- Related PDB Entries:6JOD
Kimura KT, Asada H, Inoue A, Kadji FMN, Im D, Mori C, Arakawa T, Hirata K, Nomura Y, Nomura N, Aoki J, Iwata S and Shimamura T. (2019)- Structures of the 5-HT2A receptor in complex with the antipsychotics risperidone and zotepine
- Nat.Struct.Mol.Biol. 26: 121-128
- DOI:10.1038/s41594-018-0180-z. PubMed ID: 30723326
- Related PDB Entries:6A93,6A94
Morimoto K, Suno R, Hotta Y, Yamashita K, Hirata K, Yamamoto M, Narumiya S, Iwata S and Kobayashi T. (2019)- Crystal structure of the endogenous agonist-bound prostanoid receptor EP3
- Nat Chem Biol.15: 8-10
- DOI:10.1038/s41589-018-0171-8. PubMed ID: 30510192
- Related PDB Entries:6AK3
Toyoda Y, Morimoto K, Suno R, Horita S, Yamashita K, Hirata K, Sekiguchi Y, Yasuda S, Shiroishi M, Shimizu T, Urushibata Y, Kajiwara Y, Inazumi T, Hotta Y, Asada H, Nakane T, Shiimura Y, Nakagita T, Tsuge K, Yoshida S, Kuribara T, Hosoya T, Sugimoto Y, Nomura N, Sato M, Hirokawa T, Kinoshita M, Murata T, Takayama K, Yamamoto Y, Narumiya S, Iwata S and Kobayashi T. (2019)- Ligand binding to human prostaglandin E receptor EP4 at the lipid-bilayer interface.
- Nat Chem Biol.15: 18-26
- DOI:10.1038/s41589-018-0131-3. PubMed ID: 30510193
- Related PDB Entries:5YWY,5YHL,5YFI
- Nagarathinam K, Nakada-Nakura Y, Parthier C, Terada T, Juge N, Jaenecke F, Liu K, Hotta Y, Miyaji T, Omote H, Iwata S, Nomura N, Stubbs MT, Tanabe M. (2018)
- Outward open conformation of a Major Facilitator Superfamily multidrug/H+antiporter provides insights into switching mechanism.
- Nat Commun. 9(1):4005.
- DOI:10.1038/s41467-018-06306-x. PubMed ID: 30275448
- Related PDB Entries:6GV1
- Asada H, Horita S, Hirata K, Shiroishi M, Shiimura Y, Iwanari H, Hamakubo T, Shimamura T, Nomura N, Kusano-Arai O, Uemura T, Suno C, Kobayashi T and Iwata S. (2018)
- Crystal structure of the human angiotensin II type 2 receptor bound to an angiotensin II analog.
- Nature Structural & Molecular Biology 25(7):570-576
- DOI:10.1038/s41594-018-0079-8. PubMed ID: 29967536
- Related PDB Entries:5XJM,5XLI
- Arakawa T, Kobayashi-Yurugi T, Alguel Y, Iwanari H, Hatae H, Iwata M, Abe Y, Hino T, Ikeda-Suno C, Kuma H, Kang D, Murata T, Hamakubo T, Cameron AD, Kobayashi T, Hamasaki N, Iwata S. (2015)
- Crystal structure of the anion exchanger domain of human erythrocyte band 3.
- Science 350(6261): 680-684.
- DOI:10.1126/science.aaa4335. PubMed ID: 26542571
- Related PDB Entries: 4YZF
- Nomura, N., Verdon, G,. Kang, HJ., Shimamura, T., Nomura, Y., Sonoda, Y., Hussien, SA., Qureshi, AA., Coincon, M., Sato, Y., Abe, H., Nakada-Nakura, Y., Hino, T., Arakawa, T., Kusano-Arai, O., Iwanari, H., Murata, T., Kobayashi, T., Hamakubo, T., Kasahara, M., Iwata, S. and Drew, D. (2015)
- Nature 526; 397-401(2015)
- DOI:10.1038/nature14909. PubMed ID: 26416735
- Related PDB Entries: 4YB9,4YBQ
- Manolaridis, I., Kulkarni, K., Dodd, RD., Ogasawara, S., Zhang, Z., Bineva, G., O’Reilly, N., Hanrahan, SJ., Thompson, AJ., Cronin, N., S, Iwata., Barford, D.(2013)
- Mechanism of farnesylated CAAX protein processing by the intramembrane protease Rce1.
- Nature. 504(7479):301-305.
- DOI:10.1038/nature12754 PubMed ID: 2429179
- Related PDB Entries:4CAD,
- Hino,T., Arakawa,T., Iwanari,H., Yurugi-kobayashi,T., Ikeda-Suno,C., Nakada-Nakura,Y., Kusano-Arai, O., Weyand,S., Shimamura,T., Nomura,N., Cameron,A.D., Kobayashi,T., Hamakubo,T., Iwata,S. Murata,T. (2012)
- G-protein-coupled receptor inactivation by an allosteric inverse-agonist antibody.
Nature 482: 237-240. - DOI:10.1038/nature10750 PubMed ID:22286059
- Related PDB Entries:3VGA, 3VG9
- Haga K, Kruse AC, Asada H, Yurugi-Kobayashi T, Shiroishi M, Zhang C, Weis WI, Okada T, Kobilka BK, Haga T, Kobayashi T. (2012)
- Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist.
Nature 482:547-551. - DOI:10.1038/nature10753 PubMed ID: 22278061
- Related PDB Entries:3UON,
- Shimamura T, Shiroishi M, Weyand S, Tsujimoto H, Winter G, Katritch V, Abagyan R, Cherezov V, Liu W, Han GW, Kobayashi T, Stevens RC, Iwata S. (2011)
- Structure of the human histamine H1 receptor complex with doxepin.
Nature 475: 65-72. - DOI:10.1038/nature10236 PubMed ID:21697825
- Related PDB Entries:3RZE
- Hu NJ, Iwata S, Cameron AD, Drew D. (2011)
- Crystal structure of a bacterial homologue of the bile acid sodium symporter ASBT.
Nature 478: 408-411. - DOI:10.1038/nature10450 PubMed ID:21976025
- Related PDB Entries:3ZUY, 3ZUX
- Hino, T., Matsumoto, Y., Nagano, S., Sugimoto, H., Fukumori, Y., Murata, T., Iwata, S., Shiro, Y. (2010)
- Structural Basis of Biological N2O Generation by Bacterial Nitric Oxide Reductase.
Science 330: 1666-1670. - DOI:10.1126/science.1195591 PubMed ID:21109633
- Related PDB Entries:3O0R
- Shimamura, T., Weyand, S., Beckstein, O., Rutherford, N.G., Hadden, J.M., David Sharples, D., Sansom, M.S.P., Iwata, S., Henderson P.J.F. & Cameron, A.D. (2010)
- Molecular Basis of Alternating Access Membrane Transport by the Sodium-Hydantoin Transporter, Mhp1.
Science 328: 470-473. - DOI:10.1126/science.1186303 PubMed ID:20413494
- Related PDB Entries:2X79
